Info
1956 Nobel Physics prize for research on semiconductors and the discovery of the transistor effect
1972 Nobel Physics prize for the theory of superconductivity, usually called the BCS-theory

Info

Info
1964 Nobel Physics prize 1964 Nobel Physics prize for fundamental work in the field of quantum electronics, which has led to the construction of oscillators and amplifiers based on the maser-laser principle


1903 Nobel Physics prize in recognition of the extraordinary services he has rendered by his discovery of spontaneous radioactivity

1987 Nobel Physics prize for important break-through in the discovery of superconductivity in ceramic materials

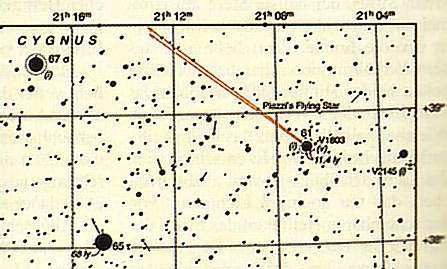
Bessel (1784-1846) was an astronomer and mathematician known best for the functions bearing his name; the Bessel functions of first and second order appear on this German stamp. Bessel was the first to measure the parallax of a star (Cygni 61) in 1838, thus making it possible to calculate its distance. Observing the motions of the stars Sirius and Procyon, he deduced that each was orbiting around another, dark star. These dark stars were later found to be white dwarfs.

Info
1967 Nobel Physics prize for his contributions to the theory of nuclear reactions, especially his discoveries concerning the energy production in stars

Biography
1948 Nobel Physics prize for his development of the Wilson cloud chamber method, and his discoveries therewith in the fields of nuclear physics and cosmic radiation

Info
1952 Nobel Physics prize for the development of new methods for nuclear magnetic precision measurements and discoveries in connection therewith


Info
1975 Nobel Physics prize for the discovery of the connection between collective motion and particle motion in atomic nuclei and the development of the theory of the structure of the atomic nucleus based on this connection
Oct. 7 1885 - Nov. 18 1962

1922 Nobel Prize for his services in the investigation of the structure of atoms and of the radiation emanating from them
Bohr is known primarily for his pioneering work in the field of atomic theory. He was born in Copenhagen and was educated at the University of Copenhagen at the time when Max Planck had just begun the development of quantum mechanics. After completing his dissertation on the electron theory of metals in 1911, Bohr went briefly to Cambridge and then on to Manchester, England. There he worked under Ernest Rutherford, who in 1911 had published the theory that the atom consisted of a central nucleus orbited by electrons. The problem with this model was that, according to classical electrodynamic theory, the electrons should radiate and therefore lose energy and spiral into the nucleus. With the problem of atomic structure in mind, Bohr worked in Manchester relentlessly from May through July of 1912 and succeeded in obtaining a formula correctly describing the absorption of helium nuclei by other atomic nuclei. His guiding notion was that the energy lost by a helium nucleus in flying through an atom depends not on the size of the atom but rather on the distances between the nucleus and the various electrons in the atom. In the autumn of 1912, Bohr returned to Copenhagen to become an assistant at the university and began a happy marriage that produced six sons. He pondered the results of his work at Manchester and in 1913 published a crucial trilogy of papers that made a deep impression on Albert Einstein and other scientists. Especially astonishing was that Bohr, in his explanation of atomic structure, departed from classical mechanics and made use of Planck's constant and the quantum theory. The result was a model of the atom in which radiation was emitted only when an electron jumped from one quantum orbit to another. The frequency of the light emitted by the atom was thus not related to any frequency in the atom; rather, it was connected with the difference between two energy levels within the atom. At the age of 28, Bohr had reached the summit of his career with his theory of the atom. In 1916, Bohr was appointed professor of theoretical physics at the University of Copenhagen, and in 1922 he was awarded the Nobel Prize for physics. In the following decades he continued to work on the implications of his theory, notably putting an earlier knowledge of surface tension to use in his "droplet model" of the nucleus, which treats the nucleus as if it were a water droplet held together by its surface tension. In 1939, one year before the German invasion of Denmark, Bohr became president of the Royal Danish Academy of Sciences and Letters and began to develop a theory of nuclear fission. In 1943 the Germans planned to arrest him and make him work in Germany on an atomic project, but Bohr fled with his family and spent the war years in the United States, where he participated in the British-American atomic bomb project at Los Alamos. After the war Bohr returned to Denmark.
Born: 20 Feb 1844 in Vienna, Austria
Died: 5 Oct 1906 in Duino (near Trieste), Austria (now Italy)

Boltzmann is best known on his invention of statistical mechanics he established independently of Willard Gibbs. Their theories connected the properties and behaviour of atoms and molecules with the large scale properties and behaviour of the substances of which they were the building blocks.
Biography

1954 Nobel Physics prize for his fundamental research in quantum mechanics, especially for his statistical interpretation of the wavefunction
Born (1882-1970), a German theoretical physicist, was a pioneer in developing quantum mechanics. In collaboration with his students and assistants Werner Heisenberg, Pascual Jordan, and Wolfgang Pauli, he attempted to develop a new quantum mechanics. When Heisenberg succeeded in 1925, Born and others were able to advance the theory, using more systematic and powerful mathematics.

Boscovic, a Croatian Jesuit developed the first coherent description of atomic theory which is one of the great attempts to understand the structure of the universe. What is remarkable is that his works appeared well over a century before the birth of modern atomic theory, and his influence on modern atomic physics is evident from the number of scientific articles still written about him.
Born: 1 Jan 1894 in Calcutta, India
Died: 4 Feb 1974 in Calcutta, India

Bioghraphy
Bose was able to derive Planck's equation from quantum mechanics as applied to photons. His collaboration with Einstein produced Bose-Einstein statistics which have found wide application in describing the behavior of particles with zero or integral spin that are now known as bosons, as opposed to fermions (electrons, protons, etc.) Multiple bosons may occupy the same energy state; the Pauli exclusion principle does not apply.

1954 Nobel Physics prize for the coincidence method and his discoveries made therewith. OTHE, Walter Wilhelm Georg (1921-1957) German physicist. - He died February 8, 1957 in Heidelberg.

Boyle is called by some the Father of Chemistry. His science sprang directly from his faith. All of his writings show the imprint of Christianity. As a young man, newly converted to Christ, he struggled with faith because the science of the day contained so much which was contrary to his belief. He therefore determined that every fact must be clearly established and tested, in which case he felt certain that it would prove compatible with scripture since both had the same author.

Info

Info

Brahe, an astronomer, observation of an exploding star, De Nova Stella, gives us the word "nova." His many observations were passed on to his assistant Kepler, who used them to derive his three laws of planetary motion in a heliocentric universe. For over 20 years Tycho made observations from his castle Uraniborg on the island of Ven, given him as a fief by the king to keep the famed astronomer in Denmark. Brahe completed the first eight chapters of De mundi aetherii recentioribus phaenomenis, a book on the comet of 1577, in which he showed that the comet "was beyond the Sun [an impossibility in the Aristotelian view] and that its orbit must have passed through the solid celestial spheres, if these existed" (Crombie 1952:314). In the ninth chapter, he offers a new system in which the Earth is immobile and the planets, except for the Earth, revolve around the Sun, thus rejecting both Ptolemy's and Copernicus's systems. This was published in 1588.


1909 Nobel Physics prize in recognition of the contribution to the development of wireless telegraphy. Braun was born June 6, 1850 in Fulda, Hesse-Kassel, Germany and died. April 20, 1918 in Brooklyn N.Y. USA. In 1897, on February 15th, Braun published in the journal "Annalen der Physik und Chemie" his research results on a method to record and study the time dependence of alternating currents. He invented and developed the so-called "Braun tube" as a fast responding recording instrument, the first cathode-ray oscilloscope. Cathode-ray tubes had previously been characterized by uncontrolled rays; Braun succeeded in producing a narrow stream of electrons, guided by means of alternating voltage, that could trace patterns on a fluorescent screen. Ferdinand Braun studied the design of Marconi's transmitter, which had the spark gap connected directly between the antenna and ground. To increase the range of the underwater telegraphy system Braun changed the original circuit which also had the antenna directly coupled to the spark discharge. In Braun's improved arrangement, a primary coil was placed in the oscillation producing spark gap circuit. That coil and a loosely coupled secondary coil were used to transfer energy to the antenna. The effective communication range of the underwater system had increased even more when both the oscillator circuit and the antenna circuit were in resonance. The "loose" coupling, which Braun now used between the spark-gap oscillator circuit and the antenna circuit, produced considerably less damping of the pulses of oscillations. The effect of low damping was highly beneficial in that much more energy was radiated and the energy was distributed over a much narrower range of frequencies. Making the two circuits resonant further increased the amount of energy transferred to the antenna. Braun's use of loose coupling between the oscillator and antenna circuits of a conventional wireless transmitter dramatically increased the range of aerial (i.e., through air) transmissions. This was demonstrated by Braun in 20-9-1898. Within a month of his initial test Braun predicted that his aerial wireless telegraphy equipment now would be able to span distances of 100 km. Braun's improvements effectively eliminated Marconi's previous patent monopoly on wireless telegraphy. The new company, which would be known as "Telebraun," was formed and the company filed an application for a patent on Braun's circuit. The company was later called "Telefunken."

Russian astronomer, educated at home and at the Richelieu College in Odessa, he entered the University of Moscow in 1851. There he devoted himself to the study of astronomy and after his graduation he continued to study at the University while working at the observatory. In 1857 he was appointed assistant professor of astronomy at the University of Moscow and in 1865 he became a full professor there. In 1867 he went abroad, spending over a year in Italy. From 1873 to 1876 he was the dean of the physical-mathematical faculty at the University of Moscow Observatory. He edited eleven volumes of the Annals of the Moscow Observatory. His principal work was the investigation of the form of comets in connection with his theory of meteors. He founded the first Russian School of Astronomy at the University of Moscow. In 1890 he accepted the post of director of the Pulkovo Observatory. In 1895 he found it was necessary for him to relinquish this job for reasons of ill-health. in addition to belonging to the Moscow Mathematical Society and the Russian Astronomical Society, he held membership in numerous scientific scoieties abroad. He is well-known for his research on comets. Two of his monographs on this subject are: O khvos-takh komet (On the Tail of Comets, 1862); and Vozmuschchenia komet, mezavisyashcie ob planetnykh prityazheny (Comet Perturbations Which are not Caused by Attractions by the Planets; doctoral dissertation, 1863). He died in St. Petersburg May 1904.

1946 Nobel Physics prize for the invention of an apparatus to produce extremely high pressures, and for the discoveries he made therewith in the field of high pressure physics
1994 Nobel Physics prize for pioneering contributions to the development of neutron scattering techniques for studies of condensed matter for the development of neutron spectroscopy

French physicist; brother of Louis Victor, duc de Broglie. His contributions include notable work on X rays and in atomic physics, radioactivity, and electricity. He became a member of the Academy of Sciences in 1924 and of the French Academy in 1934
Born: 15 Aug 1892 in Dieppe, France
Died: 19 March 1987 in Paris, France

1929 Nobel Physics prize for his discovery of the wave nature of electrons. Broglie provided a relation that elementary particles should exhibit wave-like characteristics, just as under certain circumstances light waves assume the properties of particles.
Biography